Intro to the Tuttnauer T-Edge Pre-vacuum B-Class Steam Sterilizer Leave a comment
The Tuttnauer T-Edge Pre-vacuum B-Class Steam Sterilizer has arrived and boy is it turning some heads. In this article we want to lay out the basics to give you some insight into this incredible autoclave*. And if you just want to talk to someone, call our Free Tech Support at 704-966-1650 Option 3.
What is the Tuttnauer T-Edge?
The Tuttnauer T-Edge Pre-vacuum B-Class Steam Sterilizer is designed for sterilization of medical and surgical goods such as wrapped and unwrapped solid, hollow, and porous loads used in dental and medical clinics, first aid rooms, hospitals, and laboratories etc.
The T-Edge Table-top autoclave is a fully automatic sterilizer. A computerized control unit ensures a fully automatic sterilization cycle, control and monitoring of physical parameters, and clear documentation of the sterilization cycle. Drying is performed with the door closed.
T-Edge Specifications
Device Overall Dimensions
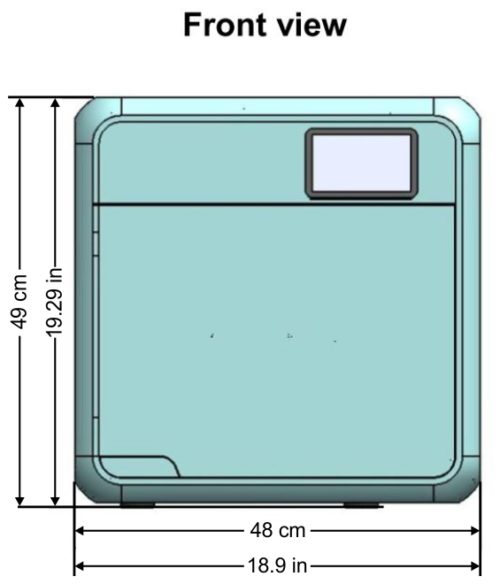
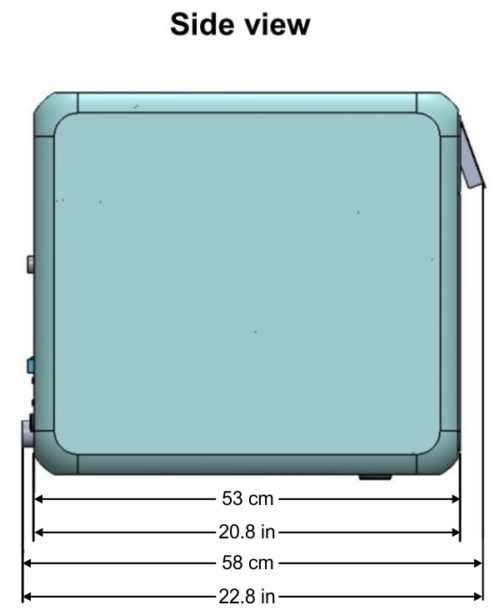
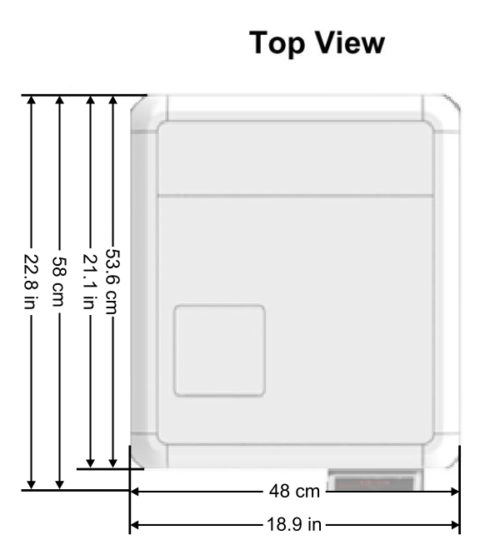
Device Properties
| Property | Dimension | |
|---|---|---|
| External Size | Width | 48cm / 18.9in |
| Height | 49cm / 19.29in | |
| Depth | 58cm / 22.8in (supporting common install base carry a 60cm counter top) | |
| Chamber | Diameter | 25cm / 9.8in |
| Depth | 46cm / 18in | |
| Volume | 23lit / 6gal | |
| Usable Chamber Space | 75% (~17 L) | |
| Max. Allowable Working Pressure (MAWP) | 2.8 bar | |
| Maximum load per item | 0.2kg / 0.44lbs | |
| Maximum load per tray | Unwrapped | 1.2kg / 2.6lbs |
| Wrapped | 0.72kg / 1.58lbs | |
| Maximum Solid load | Unwrapped | 6kg / 13.2lbs |
| Wrapped | 3.5kg / 7.7lbs | |
| Maximum textile load | 1.5kg / 3.3lbs | |
| Tray Dimensions | cm 42.1 x 18.9 x 2.05 in 16.5 x 7.4 x 0.8 |
|
| No. of trays | 5 | |
| Net Weight | 50kg / 110lbs | |
| Shipping Weight | 60kg / 132lbs | |
| Floor loading requirements | 75kg / 165lbs | |
| Mineral-free water reservoir | Max. water volume | Overlow (up to the float) -- 3.8lit / 1gal |
| Min. water volume | 1lit / 0.26gal | |
| The volume used by the sterilization cycle/load having the highest steam consumption | Recorded 800ml were required to sterilize full load of porous type using "Wrapped 121". | |
| Recorded 1400ml were required to sterilize full load of wrapped instruments/porous type using "Prion 134". | ||
| Used (waste) water reservoir | Max. water volume | Max vol. - 4lit / 1.05gal Float - 3.7lit / 0.9gal (max allowed for start cycle) |
| Safety relief valve | 40 PSI | |
| Load No. counter | Counting from 0 to 999 and nullifies. | |
Device Electrical Data
| Property | Value |
|---|---|
| Total Power | 2000W |
| Voltage | 1 PH / 230 VAC |
| Amperage | 10A |
| protection against electrical shock | Class I (IEC 60601-1) |
| Mains supply fluctuation | +/- 10% |
| Frequency (Hz) | 50Hz |
T-Edge System Parts
Front View
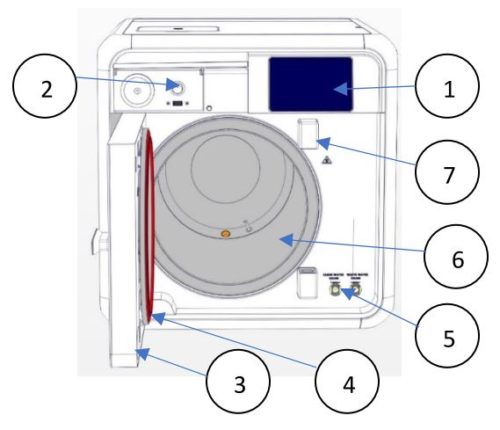
| No. | Description |
|---|---|
| 1 | Touch Screen |
| 2 | On/Off Switch |
| 3 | Chamber Door |
| 4 | Door Gasket |
| 5 | Mineral-free (left) and Waste Water (right) Reservoir Drains Chamber |
| 6 | Chamber |
| 7 | Door Switches |
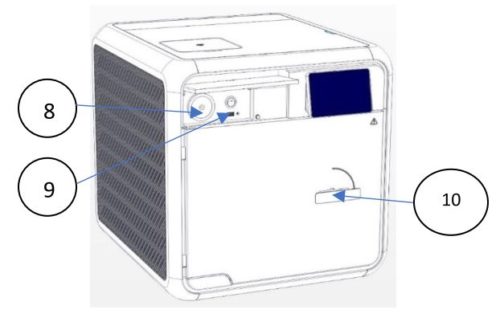
| No. | Description |
|---|---|
| 8 | Air Filter |
| 9 | USB Socket |
| 10 | Door Handle |
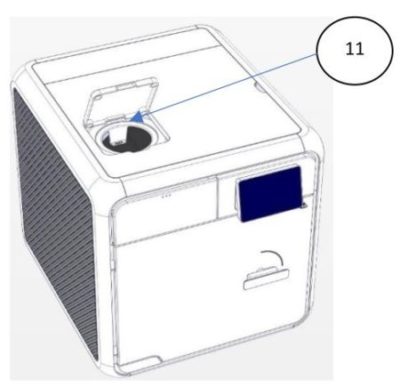
| No. | Description |
|---|---|
| 11 | Mineral-free Water Reservoir Opening |
Rear View
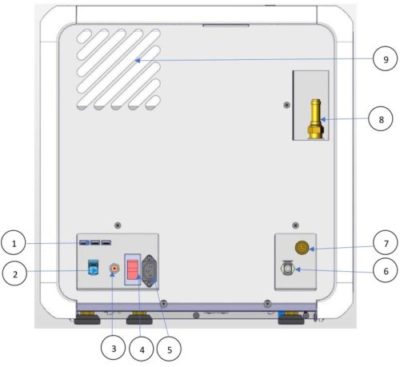
| No. | Description |
|---|---|
| 1 | USB Ports |
| 2 | LAN Socket |
| 3 | Cut-off Thermostat |
| 4 | Circuit Breaker On/Off Switch |
| 5 | Power Socket |
| 6 | Waste Outlet |
| 7 | Mineral free inlet (available in AMS10-230-W-T/ AMS10-230-W-PED-T only) |
| 8 | Safety valve |
| 9 | Aeration Ventilation opening |

Installation Instructions
Lifting and Carrying

Before moving the autoclave, make sure that the electric cord is disconnected from the power, and there is no pressure in the chamber. Drain the water from the reservoir. Do not drop the device!
To avoid injuries, lifting and carrying should be done with at least two persons or by using a fork-lift or any other mechanical aid.
Device Placement and Operating Conditions
- The autoclave is intended for indoor use only.
- Check and verify that the counter carrying the autoclave is a rigid and leveled sruface and can carry a load of 75Kg / 165Lbs.

- Check and verify that the dimensions of the surface of the counter are at least 55cm x 60cm (21.6in x 23.6in)
- Keep the back and the sides of the autoclave approximately 10cm / 4in away from the wall to allow ventilation and facilitate the device disconnection.
- If placed in a cabinet, verify that the rear of the cabinet is open to allow ventilation.

- It is recommended that enough space be left around the autoclave to give a technician access for servicing the machine.
- Check and verify that the ambient temperature range is 5°C-40°C / 41°-104°F. It is preferable not to exceed 30°C / 86°F.
- Check and verify that the ambient relative humidity does not exceed 80%.
- The operational altitude shall not be over 4000 meters.
- Ambient pressure shall not be lower than 60.5 KPa (if the altitude and temperature are kept in the manufacturer’s instructed ranges above, and no forced extreme negative pressure is applied near the autoclave, then ambient pressure of 60.5 KPa or higher is guaranteed, as it is a function of altitude and temperature).
- Operate the autoclave only in the manner specified in the manual. If the equipment is used in a manner not specified by the manufacturer, the protection provided by the equipment may be impaired.
Connections to Utility Supplies
- Check and verify that the power supply is a 1 phase, 230Vac ±10%, 50Hz, 10A supply.
- Check and verify that the electrical net is protected by a current leakage safety relay.
Storage
After the removal of the autoclave from the package, Tuttnauer recommends the following:
- Keep the device dry.
- Keep the device away from sunlight and protect it from heat.
Initial Operation of the Device

- Plug the power cord into the power socket
- Turn on the ON/OFF switch mounted on the bottom left side of the front panel (see the front view above)
- When you turn on the autoclave, it will automatically warm up.

- Fill the Mineral Free Water Reservoir with water meeting the quality specs.
- Set date and time
Please note: Prion program requires the demineralized water level in the reservoir to be filled to the maximum level in order to start the program, otherwise an alert will be prompted “please fill water tank to full for start”.
Pre-sterilization Cleaning and Disinfection of Instruments and their Loading into the Device

The most important stage begins with removing debris by cleaning and rinsing. Effective cleaning is affected by several factors: water quality, type, concentration, and quality of a cleaner, effective washing method, and adequate rinsing and drying.
Cleaning dried blood is especially difficult because it flows and dries in difficult-to-clean locations. It shall be washed away. Mechanically scrubbing, high pH detergents, enzymatic solutions, and water spray at high pressure will this contamination.
Note: Consult the Medical Device manufacturer relating adequate and most effective cleaning method and cleaning agents.
Instruments which are composed of several components shall be dismantled.
Disinfection is the next step. It is important6 for safe handling. There are various methods and means for disinfection like soaking in liquid chemical disinfectants or hot water disinfection.
Packaging. The target in packing medical items is to assure that the contained goods are sterile and maintaining them sterile till opening the package.
There are various methods and techniques used in the preparation and packaging of surgical instruments.
Check the instructions of the item manufacturer as t the proper procedure for cleaning and sterilizing each item. The item manufacturer’s instruct5ions always supersede any other instructions.
- Clean instruments immediately after use to remove any residue. It is recommended that all instruments be ultrasonically cleaned using Tuttnauer’s Clean & Simple enzymatic cleaning tablets or other suitable solution.
- After cleaning, rinse instruments under tap water for 30 seconds and pat or air dry to remove residual minerals. If your tap water has a high mineral content, then rinse a secod time in a bath of distilled water to remove minerals and pat dry.
- Launder textile wraps prior t sterilization, thoroughly rinse wraps laundered in chlorine bleach. Chlorine bleach can harm your stainless-steel instrument and the sterilizer.
- Follow the instrument manufacturer’s instructions on the use of products for cleaning and lubricating instruments that have been ultrasonically cleaned.
- Be sure that instruments of dissimilar metal (stainless steel, carbon steel, etc.) are separated. Carbon steel instruments should be bagged or placed on autoclavable towels and not directly on stainless steel trays (mixing will result in damage to the instruments or trays from the oxidation of these materials).
- Load items within the boundaries of the tray so that they do not touch the chamber walls or fall off when the tray is moved. Items should not be allowed to touch the walls of the Chamber as the hot metal can damage the item.
- Don’t overload the Sterilizer trays (see Specification). Overloading will cause inadequate sterilization & drying.
- Make sure that all instruments remain apart during the sterilization cycle. Surfaces that are hidden because items are covering other items will not be exposed to the steam and will not be sterilized.
- Disassemble or sufficiently loosen multiple-part instruments prior to packaging to permit the sterilizing agent to come into contact with all parts of the instrument.
- Verify that packaging methods are in accordance with the good practice approach and the packaging materials used are in agreement with applicable standards.
- Tilt on edge items prone to entrap air and moisture, e.g. hollowware, so that only minimal resistance to removal of air, the passage of steam, and condensate will be met.
- Wrapped instruments should be placed in material which will allow steam penetration and promote drying, such as autoclave bag, autoclave paper, or muslin towels.
- When loading pouches on the tray, put them paper side up. nylon side toward the tray (see the figure below)

- Tubing should be rinsed after cleaning. When placed in the tray, make sure that both ends of the tubing are open and there are no sharp bends or twists.
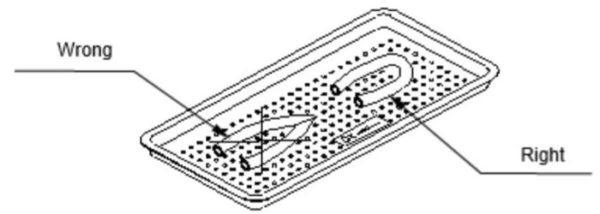
- Cassettes should be placed on the tray rack in place of the trays. They should not be touching each other or the Chamber walls. There should be about 2.5cm / 1 in. between cassettes or packs for proper steam circulation.
- If spotting is detected on the instruments it is necessary to determine if the spot is dirt or rust. The first step would be to use an ordinary eraser t remove the spot. If there is no pitting under the spot, then the spot is only dirt. Dirt spots on an instrument may be an indication that the autoclave needs to be cleaned or that the instruments were not adequately cleaned or dried prior to sterilization. If removal of the spot reveals pitting, then the spot is most likely rust. Rust spots on an instrument are not uncommon on inexpensive instruments. It may also be an indication that the instruments were rinsed in tap water with a high mineral content. These minerals when exposed to high temperatures and steam will accelerate the oxidation of the metal. One suggestion would be to final rinse the instruments in a distilled water bath and pat dry to absorb residual water and minerals.
- If the instruments exhibit a discoloration this can be due to the mixing of carbon steel and stainless steel. When these two metals come into contact with each other electrolysis occurs that breaks down the metal. The best solution is to separately wrap the carbon steel instrument to insulate it from other instruments on the tray and the tray itself.
For more information on the operation of the Tuttnauer T-Edge see our other blog posts to include:
Tuttnauer T-Edge Operating Instructions
Tuttnauer T-Edge Control Panel Review
To see the Tuttnauer T-Edge you can visit the following:
You can see the Tuttnauer T-Edge 10B 220v 10″X18″ Chamber OEM T-EDGE10B here
You can see the Tuttnauer T-Edge 10B 220v 10″X18″ Chamber OEM T-EDGE10S here
*All information taken from Tuttnauer Operation and Maintenance Manual – Pre-vacuum B-class Steam Sterilizer T-Edge – Man205-0502001EN rev G – May 2020
As always if you have any questions about this process or anything else please feel free to contact us and take advantage of our “FREE TECH SUPPORT.”
We also offer FREE VIRTUAL TECH SUPPORT to “See and Talk” with a “Real Time Live Technician” for any problems you may be in need of help with.
You can also use our “FREE MAINTENANCE PROGRAM”. Take the guesswork and worrying about what unit is due for maintenance and which maintenance cycle it is time for. We will keep track of all your autoclaves and let you know when it’s time for anything.


