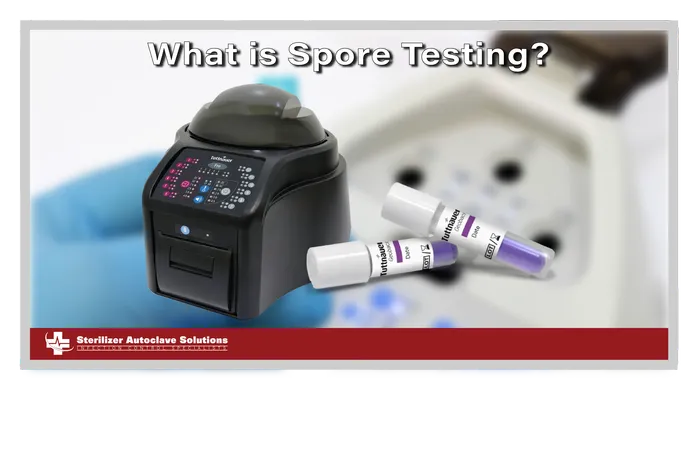
What is Spore Testing?
In the world of patient care, nothing is more critical than ensuring the absolute sterility of medical instruments. While many steps in the infection control chain are vital, only one step offers definitive, biological proof that dangerous pathogens have been destroyed… that’s what we turn to. Years of study that have culminated into the penultimate sterilization testing method: biological monitoring. More specifically, one facet of biological monitoring in the form of spore testing. Because a screen can show you something, but only when you bring the investigation to the source, can you truly find the right answers.
So in this article, we’d like to answer some questions about this testing method, spore testing. We’ll go into detail about spore testing itself, the importance of it, and some other questions that will demystify as much as possible about why this method is so effective. We’ll be asking quite a few questions here, so we hope you’re ready to take some notes.
What is a Spore Test?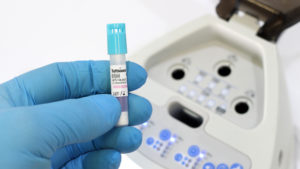
We can’t begin a full lesson without starting at the beginning, now can we? Spore testing, also known as biological monitoring, is the only verifiable method used to confirm that a sterilization process has successfully killed all microbial life, including highly resistant bacterial spores. It is the gold standard for sterility assurance in healthcare. Spore testing utilizes a vial known as a biological indicator, which is used in an autoclave cycle to test the efficacy of your unit. The test vials contain samples of certain bacterial spores, usually specific, non-pathogenic bacterial spores, such as Bacillus, Geobacillus, and Clostridium.
These spores are chosen because they are much more difficult to kill than common infectious bacteria and viruses. They’re taken through the cycle, and the outcome of the test hinges on if they survive or not. By proving that the sterilizer can kill the toughest organisms, you effectively know that it can neutralize any other kind of pathogen you’d run into. In essence, spore testing verifies the lethality of the entire sterilization cycle.
Why is Spore Testing So Important?
Learning the importance of spore testing not only shows you why it’s something that should be commonplace in any practice… But it should also serve as a form of encouragement to continue using it.
Spore testing is the ultimate safety net for infection prevention. Especially when it comes to your reusable tools. While healthcare facilities can rely on chemical monitoring or even mechanical monitoring — like checking the gauges and using color-changing test indicators inside the unit… These various methods only show that the cycle specifications are up to code. Your autoclave can show as many numbers on a screen as it wants, but these methods do not prove that the unit has effectively killed off the microorganisms.
A spore test, however, uses biological monitoring, which tells you exactly what you need to know. These tests are supposed to be performed on a weekly basis, and are a vital part of risk management. A failed spore test means that immediate action must be taken. Your autoclave should be decommissioned temporarily to squash the chance of any possible contamination. Because all it takes is one compromised load to come out of that faulty unit… then a preventable, easy fix like a door gasket can turn into a new headache all too quickly.
How Do You Perform a Spore Test?
Performing a spore test is a straightforward process involving placing a biological indicator in a load of instruments to run it through a sterilization cycle. The process will look something like this:
1.) Placement: The indicator is placed in the area of the sterilizer chamber that is considered the most challenging for steam or heat penetration (often the center of the chamber or inside a challenging tray/pouch).
2.) Cycle Run: The normal sterilization cycle is run.
3.) Incubation: After the cycle, the exposed indicator is removed and placed into a specialized incubator for the required incubation time, like the Tuttnauer BioNova Biological Incubator (shown right). These devices are the ones that test the vials to give you your results.
4.) Result Interpretation: Once the biological reader is finished, your result will often show through a specific indicator, like an LED light or printout depending on the reader. The result, if negative, means your sterilizer is in top shape, ready to tackle the work ahead.
If it comes back positive, that means that your autoclave has something preventing it from achieving proper sterilization. And that itself is usually an indicator of a problem that needs to be solved. So if you return a positive spore test, discontinue the use of your autoclave until it can return a negative result.
What is a Biological Incubator?
So many questions, right? Well we hope you’re still with us, because they’re all important – including this one.
A biological incubator, is a highly specialized, temperature-controlled device that’s used to replicate the perfect environment for microbial growth. This environment is for testing the biological indicators; the vials containing the resistant spores. The vials are places in the incubator, then they’re “challenged” in a way. The vials contain highly resistant spores, so when the incubator gets going, the test of survival is what dictates the result.
A successful result is confirmed when the vial comes back where no growth occurred. But if the indicator vial shows any signs of cloudiness or other visual signs, that means that the spores successfully began to thrive. Which means that the test is a failure. Everything sterilized in that cycle are immediately recalled, and the investigation of what’s stifling your autoclave begins.
Final Thoughts
Ultimately, the goal of sterilization is absolute microbial destruction, and spore testing is the only method that provides verifiable proof. While mechanical gauges and chemical indicators offer important checks, they’re just data for showing effective heat and pressure.
By intentionally challenging your sterilizer with the most resistant bacterial spores, biological monitoring eliminates all doubt, acting as the final, non-negotiable step in quality assurance. Consistently performing this simple test is how you confirm compliance, protect your patients, and ensure that your instruments are truly safe.
So if you have any questions about spore testing, biological indicators, or anything else, give us a call at 704-966-1650 Option 3, and our technicians can help you. You can also find links below to our various programs to help you with things like preventative maintenance, and more.
As always if you have any questions about this process or anything else please feel free to contact us and take advantage of our “FREE TECH SUPPORT.”
We also offer FREE VIRTUAL TECH SUPPORT to “See and Talk” with a “Real Time Live Technician” for any problems you may be in need of help with.
You can also use our “FREE MAINTENANCE PROGRAM”. Take the guesswork and worrying about what unit is due for maintenance and which maintenance cycle it is time for. We will keep track of all your autoclaves and let you know when it’s time for anything.