On/Go Rapid Antigen Kit
Intrivo On/Go Covid-19 Rapid Antigen Home Test Kit – 1000 Tests RCPM-00279
Availability:
Out of stock
FREE SHIPPING!
$10,500.00 $10,815.00
Out of stock
Intrivo’s AI-powered, full-stack COVID management solution combines:
- Best in class diagnostics
- Leading edge technology
- Delightful user experience
- Robust security/privacy
- Machine learning…to keep employees, staff, and customers safe and healthy
Developed by Intrivo’s Silicon Valley experts, approved by Apple & Google
Included in Purchase:
- 1000 Test Devices
- 1000 Assay Buffers
- 1000 Extraction Vials and Caps
- 1000 Specimen Collection Swabs
- 500 Positive and 500 Negative Control Swabs
- 500 Instructions for Use
Key benefits:
- 10 minute test read
- Artificial intelligence to support result reading
- Guided process reduces errors
- Inbuilt communication platform
- Fully secure and protective of privacy, built for accessibility
Best in class clinical performance, FDA authorized
- US made, accurate test, high volume, competitive price, lower nasal comfort
- Impactful consumer branding and marketing
- Companion native app with delightful user experience that boosts accuracy…offer fastest, most accurate tests at a compelling price
- Easier, automated reporting of results
- Reduced absenteeism, boosted morale, reduced liability and costs
- Easy integration with existing employer portals / apps…help employees stay safe and productive
- Live collection and processing of results data
- Specific automated response by defined cohorts (e.g. geos, stores)
- Inbuilt two way communication…avoid mass lockdowns and keep society open
Instructions For Use:
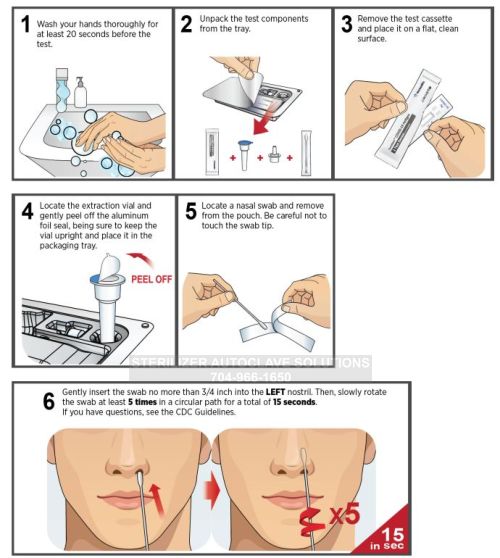
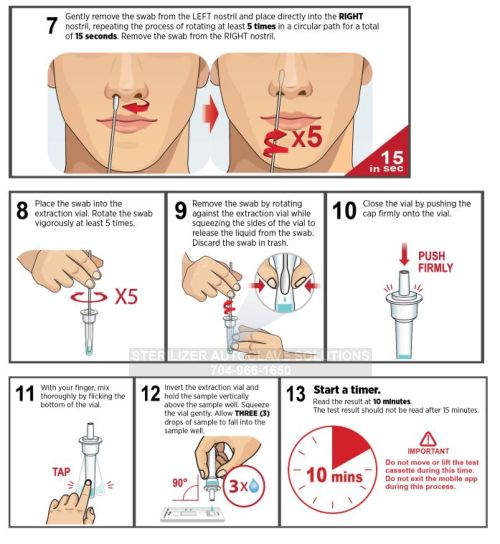
— Everything You Need to Know —
Intrivo OnGo – Instructions for Use
Intrivo OnGo – FDA Emergency Use Authorization
Intrivo OnGo – Fact Sheet for Patients
Intrivo OnGo – Fact Sheet for Health Care Providers
Intrivo OnGo – Expiration Date Extension Letter (January 2022)
Intrivo OnGo – Update to FDA EUA Labeling
Intrivo OnGo – Updated Variant Brief (Omicron)
Intrivo OnGo QRI (Quick Reference Instructions)
Intrivo OnGo Study on Variants
FDA Shelf Life Extension for On/Go
Intrivo OnGo – Packaging Files
Based on 0 reviews
Only logged in customers who have purchased this product may leave a review.
Included in Purchase:
- 1000 Test Devices
- 1000 Assay Buffers
- 1000 Extraction Vials and Caps
- 1000 Specimen Collection Swabs
- 500 Positive and 1 Negative Control Swabs
- 500 Instructions for Use









There are no reviews yet.