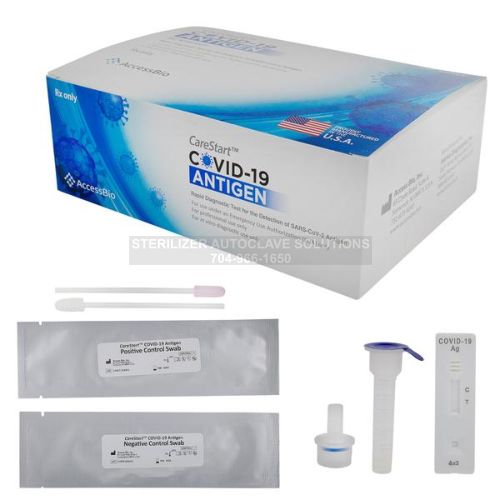
CareStart Covid-19 Rapid Antigen POC Test Kits – 10 Cases – (4000 ct) RCHM-02071
Out of stock
FREE SHIPPING!
$35,800.00 $36,874.00
Out of stock
FREE SHIPPING!
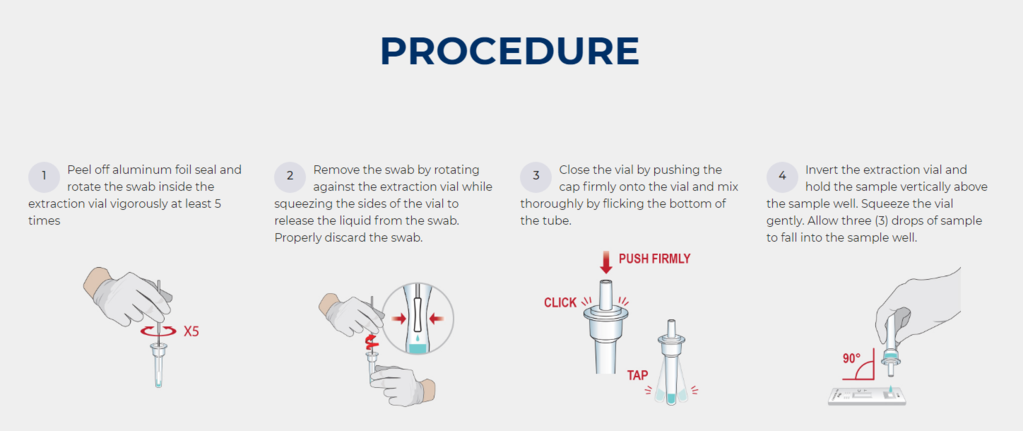
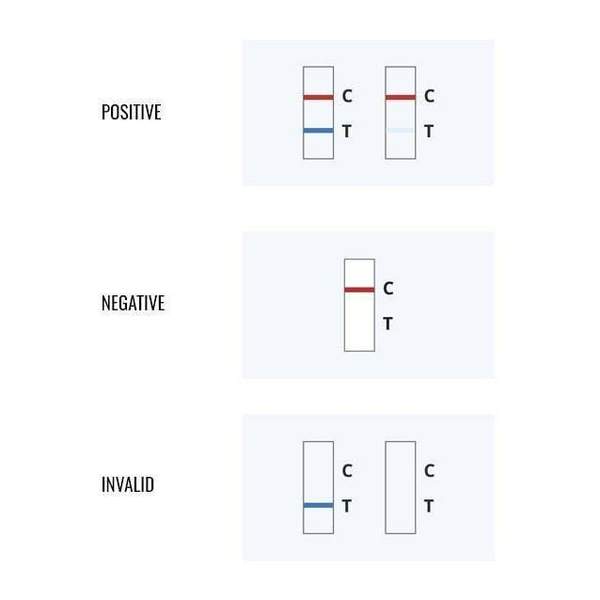
- The Ultimate POC Test With Reliable Results Within 10 minutes
- No equipment required & FDA Emergency Use Authorization & Made In USA
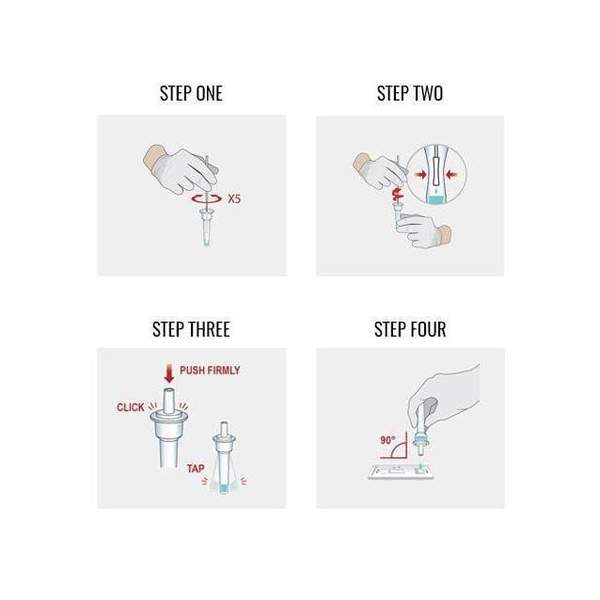
- Minimally invasive specimen collection (nasopharyngeal swabs)
- Detect SARS-CoV-2 nucleocapsid protein antigen
- Identify acute infection with 88.4% sensitivity and 100% specificity
10 Cases Includes:
- 20 Boxes per Case (Total of 200 Boxes)
- 4000 Test Devices
- 4000 Assay Buffers
- 4000 Extraction Vials and Caps
- 4000 Specimen Collection Swabs
- 200 Positive and Negative Control Swabs
- 200 Instructions for use.
Features Include:
- Rapid results in 10 minutes
- CLIA waived
- Stand-alone test kit with no equipment needed
- Designed for use in point-of-care settings
- Detect SARS-CoV-2 nucleocapsid protein antigen
- Lateral flow assay
- Minimally invasive specimen collection (nasopharyngeal)
The CareStart COVID-19 Antigen Test is a lateral flow immunochromatographic assay intended for the qualitative detection of the nucleocapsid protein antigen from SARS-CoV-2 in nasopharyngeal or anterior nasal swab specimens directly collected from individuals who are either suspected of COVID-19 by their healthcare provider within the first five days of symptom onset or from individuals without symptoms or other epidemiological reasons to suspect COVID-19 when tested twice over two or three days with at least 24 hours and no more than 48 hours between tests.
Testing is limited to laboratories certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA), 42 U.S.C. §263a, that meet the requirements to perform moderate, high, or waived complexity tests. This test is authorized for use at the Point of Care (POC), i.e., inpatient care settings operating under a CLIA Certificate of Waiver, Certificate of Compliance, or Certificate of Accreditation.
NOTE: For Serial Screening of asymptomatic individuals. The serial screening indication is only applied to products manufactured by Access Bio Inc. after April 12, 2021. The product batches listed in the Official Notification Letter (click to see) should not be marketed or used for POC serial screening purposes.
AMA CPT Codes
FDA EUA Letter
Package Insert
Antigen Fact Sheet for Healthcare Providers
Based on 0 reviews
Only logged in customers who have purchased this product may leave a review.
Sensitivity: 88.4%
Specificity: 100%
Determinations: Detection of the SARS-CoV-2 Nucleocapsid Protein Antigen
Processing Time: Approximately 10 Minutes
Storage Requirements: 34°- 86° Fahrenheit
Regulatory Status: FDA EUA Authorized
CPT Code: 87426
Depth: 6″
Width: 3″
Height: 8″









There are no reviews yet.